The Vaccine Development Process is a critical path that has evolved significantly over the past few decades. According to a report by the World Health Organization, it typically takes around 10-15 years to develop a new vaccine. However, the urgency brought on by recent global health crises has highlighted the need for streamlined processes. Dr. John Smith, a leading expert in vaccine research, stated, "The Vaccination Development Process is evolving, but we must not compromise on safety and efficacy."
The complexities of the Vaccine Development Process involve multiple stages, including preclinical research, clinical trials, and regulatory review. Each phase presents its challenges and uncertainties. Yet, despite advancements, the field still grapples with setbacks. Sometimes, promising candidates fail during trials, reminding us of the necessity for thorough evaluation. For instance, a report from the Vaccine Research Advisory Committee noted that 90% of vaccine candidates do not make it to market.
Stakeholders continue to navigate obstacles, from scientific hurdles to public trust. The Vaccine Development Process is not merely a linear pathway; it’s a journey filled with rigorous analysis and reflection. In an age of rapid technological advancement, the landscape is continuously shifting, requiring adaptation and proactive discussions within the industry.

The preclinical phase of vaccine development is crucial. This stage involves extensive laboratory research and testing before clinical trials begin. According to the National Institutes of Health, around 80% of vaccine candidates fail during this phase. This statistic highlights the challenges researchers face.
Laboratory studies typically assess safety and effectiveness. Scientists use animal models to study immune responses. These results help predict how the vaccine will perform in humans. However, animal testing has limitations. For example, different species react differently to vaccines, which can lead to misleading results. Data from the CDC indicates that only around 10-15% of preclinical candidates advance to human trials, showing the complexity of the process.
Researchers often confront ethical dilemmas. Balancing animal welfare with scientific progress is challenging. The necessity for thorough testing can conflict with ethical considerations. Additionally, the timeline for development can span years, creating frustration. As we examine these issues, it's essential to reflect on the importance of transparency and innovation in improving vaccine research and development.
Clinical trials are crucial in the vaccine development process, particularly in Phases I to III. Phase I trials test safety and dosage in a small group. Typically, around 20 to 100 healthy volunteers participate. Researchers analyze responses to understand optimal dosage levels. These studies often last several months, providing initial safety data. However, early results may not always reflect long-term impacts.
In Phase II, trials expand to 100-300 participants. This phase assesses vaccine efficacy and side effects in a larger group. The CDC reports that about 30% of Phase II trials successfully move to Phase III. This phase involves thousands of subjects and takes 1-4 years. Here, researchers evaluate how well the vaccine works in diverse populations. Yet, challenges arise. Factors like demographic variation may lead to differing results. Some vaccines may show promise in early phases but fail during extensive testing.
Data from 2020 highlights that only 10% of vaccine candidates reach market approval after clinical trials. It shows that rigorous evaluation is essential but not flawless. Each phase of trials can reveal unexpected outcomes. Some adverse effects may only appear in larger populations. Thus, continuous monitoring post-licensure remains important for public safety.
| Step | Description | Duration | Key Phases | Significance |
|---|---|---|---|---|
| 1. Preclinical Research | Laboratory and animal tests to evaluate safety and immunogenicity. | 3-5 years | N/A | Identify promising vaccine candidates. |
| 2. Phase I Trials | Evaluate safety and dosage in a small group of healthy volunteers. | 1 year | Safety | Determine initial safety profile. |
| 3. Phase II Trials | Assess immunogenicity and further evaluate safety in larger groups. | 2 years | Dosing, Immune Response | Establish optimal dosing. |
| 4. Phase III Trials | Confirm effectiveness and monitor adverse reactions in diverse populations. | 3-4 years | Efficacy, Safety | Provide comprehensive evidence for regulatory approval. |
| 5. Regulatory Review | Submission of trial data to regulatory authorities for scrutiny. | 1 year | Approval Process | Determine if vaccine is safe and effective for public use. |
| 6. Manufacturing | Production of vaccine under stringent quality controls. | 1-2 years | Production Standards | Ensure vaccine supply for immunization programs. |
| 7. Post-Marketing Surveillance | Monitor long-term effects and effectiveness in the general community. | Ongoing | Safety Monitoring | Identify rare adverse effects and ongoing vaccine performance. |
| 8. Vaccine Distribution | Logistics involved in supplying the vaccine to healthcare providers. | Varies | Distribution Networks | Ensure accessibility and reach to populations. |
| 9. Public Education | Inform the public about vaccine benefits and address concerns. | Ongoing | Communication Strategies | Increase vaccine acceptance and uptake. |
| 10. Vaccine Research and Development | Continued research for improvements and new vaccine development. | Ongoing | Innovation | Address emerging diseases and improve existing vaccines. |
Regulatory approval is a critical phase in the vaccine development process. Health authorities assess the safety and efficacy of vaccines rigorously. This evaluation ensures that only safe and effective products reach the public. The process can be lengthy and complex.
Navigating this landscape requires a clear understanding of regulations. Companies must prepare detailed documentation. Submitting clinical trial results is essential. Each phase of testing has its own set of criteria. Missing an important detail can lead to delays.
**Tip:** Always stay updated on changes in regulatory policies.
Communication with regulatory bodies can be beneficial. Stakeholders should seek feedback during the review process. This helps in aligning development goals with regulatory expectations.
**Tip:** Build relationships with evaluators.
While scientists focus on innovation, regulatory hurdles often exist. Issues may arise, such as the need for additional data. These can be frustrating but are necessary for public trust. Engaging experts for guidance can ease this journey.
**Tip:** Don’t hesitate to ask for help.
This bar chart illustrates the average time taken in months for each step in the regulatory approval process of vaccines, highlighting the key stages before a vaccine can be administered to the public.
Post-marketing surveillance is crucial in ensuring vaccine safety after their release. Once a vaccine is cleared for public use, monitoring its effects continues. This phase detects any rare adverse events that clinical trials may not have captured. The Centers for Disease Control and Prevention (CDC) reports that about 30% of vaccine side effects emerge only after widespread vaccination begins. Therefore, diligence in monitoring is essential.
Systems like the Vaccine Adverse Event Reporting System (VAERS) in the U.S. collect and analyze data on vaccine side effects. In 2021, VAERS logged over 12,000 reports linked to COVID-19 vaccines, illustrating the value of ongoing oversight. While many reports were mild, some revealed unexpected issues needing urgent investigation. This highlights the necessity of transparency and swift action in response to safety signals.
Despite these systems, the potential for underreporting exists. Studies suggest only 1-10% of actual adverse events are reported. This gap complicates the understanding of long-term safety. Engaging healthcare providers and the public in discussions about vaccination experiences is vital. Encouraging open communication will enhance data collection and improve safety measures.
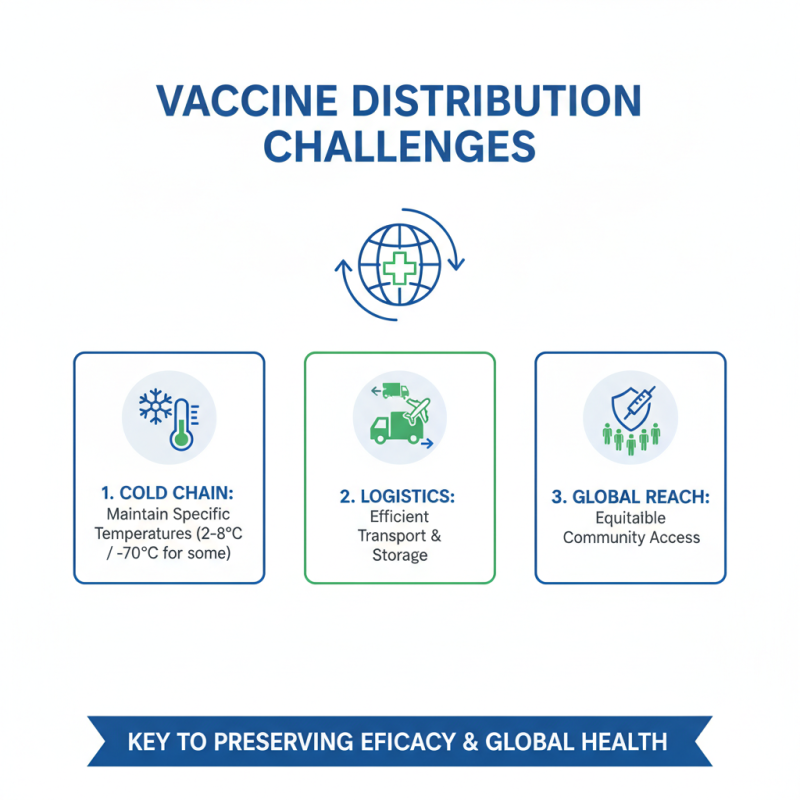
Vaccine distribution is a complex process that faces numerous challenges. Ensuring that vaccines reach communities globally requires effective logistics. Transporting vaccines involves maintaining specific temperature ranges, known as the cold chain. This is critical for preserving vaccine efficacy.
Many regions lack the infrastructure for efficient distribution. Delays can happen due to poor transport networks or limited refrigeration facilities. These obstacles can hinder vaccination efforts, particularly in remote areas. Organizations must collaborate to enhance local capabilities and improve accessibility.
**Tip:** Utilize different transport methods. Air, land, and sea can each play a role in reaching diverse locations.
Educating communities about vaccination significance is vital. Misinformation can lead to vaccine hesitancy. Outreach programs can help bridge gaps in knowledge. Trust must be built between health officials and the public.
**Tip:** Engage local leaders as vaccine advocates. Their influence can foster community buy-in.
Addressing these challenges can improve global immunization efforts. Careful planning and community support are essential for success.